Help Reduce the Risk of Bloodstream Infections
With specialty IV consumable technologies designed to provide an effective barrier against bacterial transfer and colonization and help standardize disinfection protocols, we are committed to providing tools designed to help reduce the risk of bloodstream infections and enhance patient safety.
The Challenge
Placement of a vascular access device increases the risk of bloodstream infection
Nearly all hospitalized patients have a vascular access device inserted to support their treatment, and approximately 87 percent of bloodstream infections are associated with the presence of an intravascular device.1
The CDC estimates approximately 250,000 incidents of Catheter-Related Bloodstream Infections (CRBSIs) occur annually in the United States.2 Although attributable mortality due to CRBSIs is not clear, these infections are associated with higher costs, mortality rates, and number of hospital-days.2,3
Our Solution
The design of your needlefree intravenous (IV) connectors plays a substantial role in your ability to limit hospital-acquired bloodstream infections (HA-BSIs).4
All of our needlefree IV connectors incorporate our clinically-differentiated Clave™ technology and are designed to keep patients and healthcare workers safe by providing a mechanically closed system.
Our closed needlefree connectors feature pioneering reversed split-septum, straight internal fluid path technology to help reduce the ingress and colonization of bacteria. Not only do these devices provide enhanced patient safety through innovative needlefree technology, but they have also been proven to provide an effective microbial barrier against bacteria transfer and contamination.5,6,7
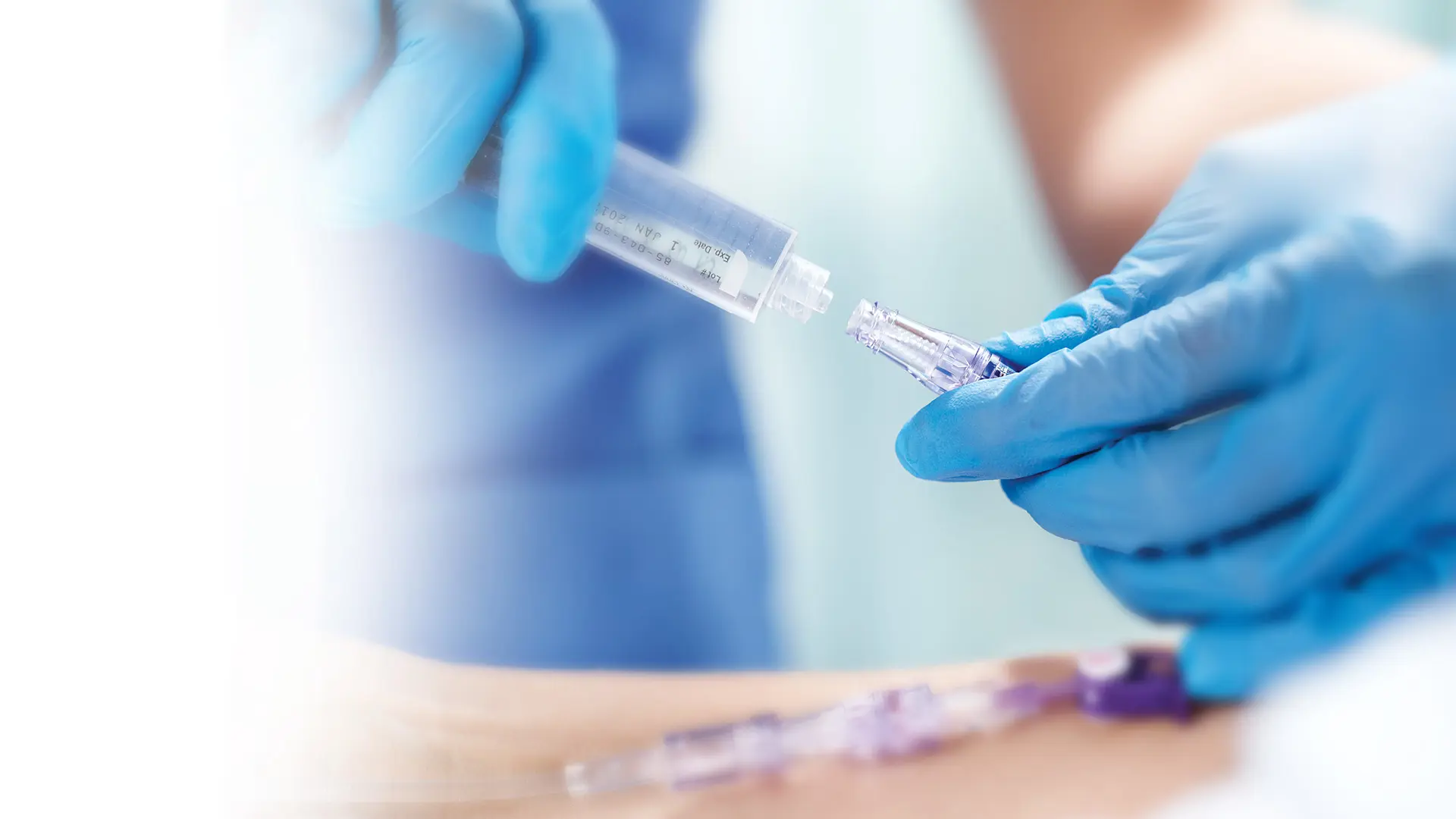
Clave Infection Control Technology
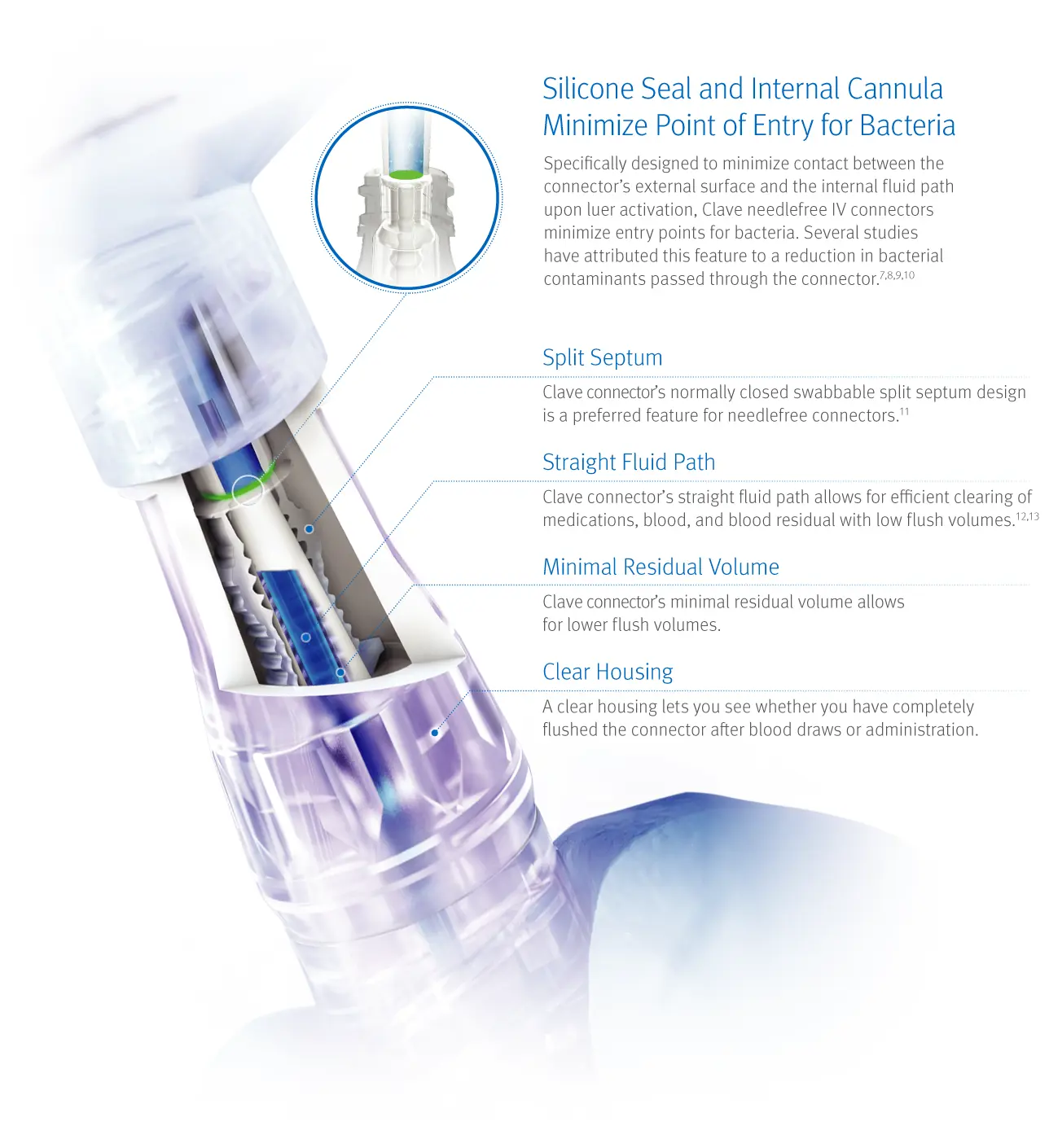
Head-to-head studies show Clave technology outperforms other connectors
In a peer reviewed comprehensive study comparing 20 different needlefree IV connectors, researchers reported ICU Medical’s connectors featuring Clave technology were “shown to have a significantly lower bacterial transfer rate than any of the other connectors tested.7
The study measured the difference among connectors in the passage rate of bacteria from the connector surface through the catheter and into the bloodstream over time, and compared biofilm formation within the connectors, catheter hub, and catheter lumen.
Bacterial transfer rate comparison of needlefree connectors
In addition to bacterial transfer at the point of vascular access, thrombus formation increases the risk of catheter-related bloodstream infections for patients. Fibrin, blood components, and biofilm can amass,13 creating a rich culture medium for bacterial growth that has been shown to result in microorganisms entering the bloodstream.14
Our Clave Neutron™ needlefree neutral displacement connector is designed to prevent fluid displacement resulting from the four known causes of displacement associated with needlefree connectors: connection or disconnection of a luer, syringe plunger compression, patient vascular pressure changes (e.g., coughing or sneezing), and IV solution container run-dry, which may cause multiple forms of reflux into a catheter.15 The Clave Neutron utilizes ICU Medical’s Clave needlefree connector technology, and has been shown to help reduce catheter occlusions by 50%.16
Warning: Clave connectors may be incompatible with some male-luer connectors including prefilled glass syringes. To avoid damage to the Clave or syringes or male luers which may result in delays of medication administration and possible serious adverse events, users should confirm mating luers or syringes have an internal diameter range of 0.062” to 0.110”. Check the internal diameter of the male-luer connector of the mating syringe prior to using it to access the Clave. Products outside of these dimensional tolerances should not be used.
Additional Infection Control Measures
Maximize infection control compliance with easy-to-use disinfecting technology
Needlefree IV connectors play an essential role in the reduction of CRBSI, but nursing guidelines still suggest that clinicians swab connectors before each access.17 Unfortunately, swabbing technique and compliance with these policies may vary, and visual confirmation of connector disinfection may be difficult.
ICU Medical SwabCap disinfecting technology can be a vital element in your efforts to help minimize infection risks and improve swabbing compliance. SwabCap’s patented disinfecting cap design has been shown to help enhance the barrier to bacterial ingress while helping you standardize disinfection protocols.18
One study showed using of SwabCap resulted in a 34% HA-CLABSI Reduction19
Infection Control Technology Designed to Help Prevent Bacterial Contamination21
Its patented thread cover design gives SwabCap the unique ability to continue disinfecting both the connector’s surface and threads for up to seven days, if not removed, for maximum safety and compliance.21
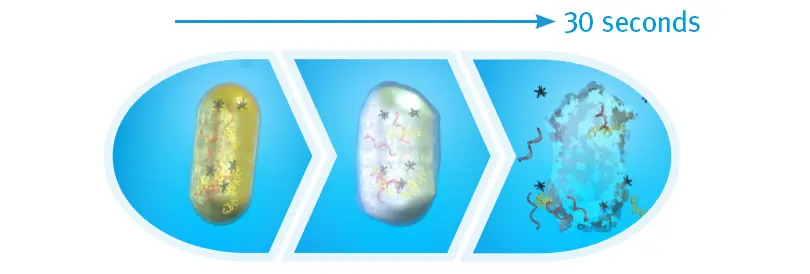
Bacterial Cell Death after 30 Seconds of IPA
When exposed to 70% isopropyl alcohol (IPA), harmful bacteria absorb the solution, making the cells swell, then breakdown and die. An in vitro study found that after 30 seonds of contact time with the cap, there were zero colony-forming units detected on the IV connectors.21

Help Reduce Hemodialysis Catheter Infections
Clinically Proven to Reduce the Rate of CLABSI in Hemodialysis Patients
ClearGuard™ HD antimicrobial barrier caps are the first and only device for sale designed to kill infection-causing bacteria inside a hemodialysis catheter hub. The caps are simple to use, yet highly effective and clinically proven to reduce the incidence of central line-associated bloodstream infections (CLABSIs) in hemodialysis patients.22,23
*Designed to kill microorganisms, not intended to be used for treatment of existing infections.
Reduce hemodialysis catheter infections with a simple, intuitive design
The ClearGuard HD cap features a rod that extends into the hemodialysis catheter hub. The rod and cap threads are coated with chlorhexidine, a well-known broad-spectrum antimicrobial agent.

- When the ClearGuard HD cap is inserted into a liquid-filled catheter, chlorhexidine elutes from the rod into the catheter lock solution
- The chlorhexidine coating dissolves to kill microorganisms on the inside and outside of the catheter hub
- The existing catheter clamp holds the antimicrobial agent inside the catheter hub between treatments
- ClearGuard HD caps are used in place of a standard cap or connector
- Designed to meet your needs, ClearGuard HD caps can be used with heparin, citrate and saline solutions
Help Protect Your Central Line Patients
Get an infection control portfolio suited for central lines
From maintaining catheter patency to guarding against bacterial ingress, the proper care and maintenance of your critical patients' central lines takes attention to detail and access to the right tools.
ICU Medical is here to help with a specialty line of infection control products tailored to the unique clinical considerations of your central-line patients.
Request More Information
References
-
Ryder, M. Catheter-related infections: It's all about biofilm. Topics Adv Pract Nurse Journal. 2005 [cited 2006 Sept 11]; 5(3). Available www.medscape.com/viewarticle/508109.
-
Centers for Disease Control and Prevention. Reduction in central line-associated bloodstream infections among patients in intensive care units-Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep 2005;54:1013-1016.
-
Blot SI, Depuydt P, Annemans L, et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis 2005;41:1591-1598.
-
Jarvis W, MD. Choosing the Best Design for Intravenous Needleless Connectors to Prevent Bloodstream Infections. Infection Control Today, August 2010 (http://www.infectioncontroltoday.com/articles/2010/07/ choosing-the-best-design-for-intravenous-needleless-connectors-to-prevent-bloodstream-infections.aspx.)
-
Ryder M, James G, Pulchini E, Bickle L, Parker A. Differences in bacterial transfer and fluid path colonization through needlefree connector-catheter systems in vitro. Presented at the Infusion Nursing Society Meeting, May 2011.
-
Moore C, RN, MBA, CIC. Maintained Low Rate of Catheter-Related Bloodstream Infections (CR-BSIs) After Discontinuation of a Luer Access Device (LAD) At an Academic Medical Center. Poster presented at the annual Association for Professionals in Infection Control and Epidemiology (APIC) Conference 2010, Abstract 4-028.
-
Ryder, M., DeLancey-Pulcini, E., Parker, A., & James, G. (2023). Bacterial transfer and biofilm formation in needleless connectors in a clinically simulated in vitro catheter model. Infection Control & Hospital Epidemiology, 1-9. doi:10.1017/ice.2023.60
-
JD Brown, HA Moss, TSJ Elliott. The potential for catheter microbial contamination from a needleless connector. J Hosp Infect. 1997.; 36:181-189.
-
Yebenes J, Delgado M, Sauca G, Serra-Prat M, Solsona M, Almirall J, et al. Efficacy of three different valve systems of needlefree closed connectors in avoiding access of microorganisms to endovascular catheters after incorrect handling. Crit Care Med 2008;36: 2558–2561.
-
Bouza E, Munoz P, Lopez-Rodriguez J, et al. A needleless closed system device (Clave™) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003; 54:279-287.
-
Guideline for the Prevention of Intravascular Catheter-Related Bloodstream Infections, Final Issue Review, 2011.
-
Data on file at ICU Medical. Low Volume Flush Characteristics of Unique Needlefree Connectors M1-1223 Rev. 1
-
Breznock EM, DVM, PhD, Diplomate ACVS, Sylvia CJ, DVM, MS, BioSurg, Inc. The in vivo evaluation of the flushing efficiency of different designs of clear needlefree connectors, March 2011
-
Ryder M. The role of biofilm in vascular catheter-related infections. N Dev Vasc Dis. 2001;2:15-25.
-
ICU Medical Clave Neutron 510(k) K100434, June 24, 2010
-
ICU Medical Study Summary. Observational In-Vivo Evaluation of the Neutron™ Needlefree Catheter Patency Device and its Effects on Catheter Occlusions in a Home Care Setting, 2011.
-
Nickel B, Gorski L, Kleidon T, et al. Infusion Therapy Standards of Practice, 9th Edition. J Infus Nurs. 2024 Jan-Feb 01;47 (1S Suppl 1):S1-S285. doi: 10.1097/NAN.0000000000000532
-
Posa P. Improving IV Connector Disinfection by Using Human Factors Engineering to Identify Effective, Nurse-Friendly Solutions. Poster presented at the APIC 4th Annual Conference. June, 2013.
-
Kamboj M, Blair R, Bell N, et al. Use of Disinfection Cap to Reduce Central-Line–Associated Bloodstream Infection and Blood Culture Contamination Among Hematology-Oncology Patients. Infection Control & Hospital Epidemiology. December 2015. 36:12.
-
Wright M, Tropp J, Schora D, et al. Continuous passive disinfection of catheter hubs prevents contamination and bloodstream infection. American Journal of Infection Control. 2012.
-
Toxicon Thirty Second Disinfection Study for SwabCap, August 2018
-
Brunelli, SM et al. Cluster-randomized trial of devices to prevent catheter-related bloodstream infection. J Am Soc Nephrol. 2018 Apr,29(4):1336-1343.
-
Hymes, JL et al. Dialysis catheter-related bloodstream infections: a cluster-randomized trial of the ClearGuard HD antimicrobial barrier cap. Am J Kidney Dis. 2017 Feb;69(2)220-227