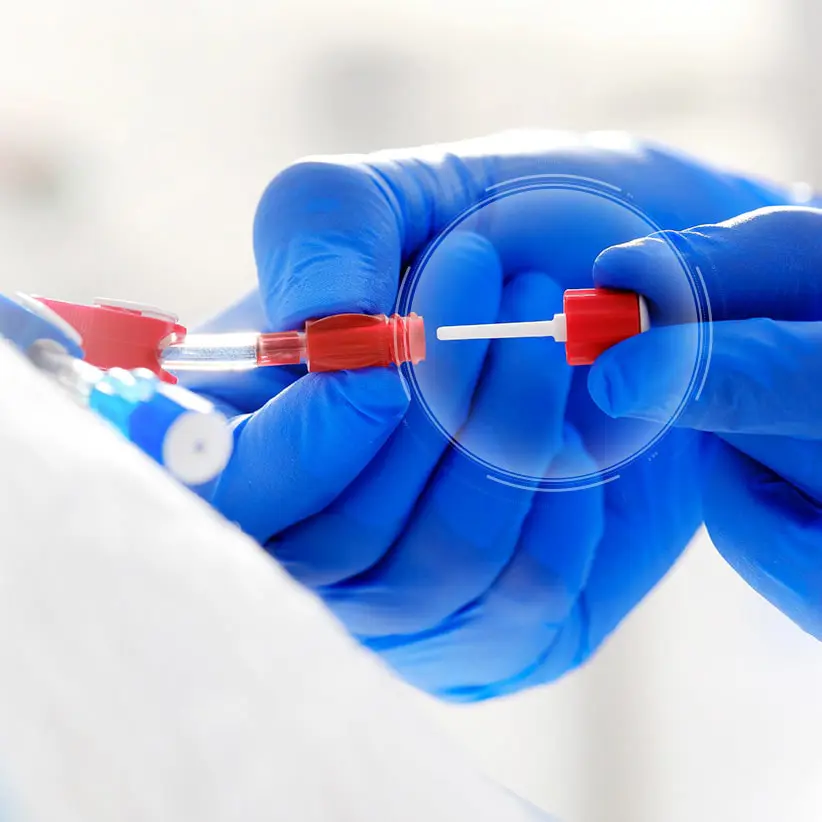
ClearGuard™ HD
Antimicrobial Barrier Caps for Hemodialysis Catheters
The ClearGuard HD caps are simple to use, highly effective and are the first and only device for sale designed to kill infection-causing bacteria inside a hemodialysis catheter hub.
*Designed to kill microorganisms, not intended to be used for treatment of existing infections.
Catheter infections are frequent, costly,
and deadly
- Catheters cause 70% of vascular access-related BSIs, and central venous catheters are used in only 19% of all dialysis procedures in the United States.1
- Central line-associated bloodstream infections (CLABSIs) are a leading cause of hospitalizations and the second leading cause of death in hemodialysis patients.1-2
- CLABSIs are very expensive, with an estimated increase in healthcare costs of approximately $27,232–$68,983, with an average of $48,108 per infection.3
Reduce hemodialysis catheter infections with a simple, intuitive design
The ClearGuard HD cap features a rod that extends into the hemodialysis catheter hub. The rod and cap threads are coated with chlorhexidine, a well-known broad-spectrum antimicrobial agent.
- When the ClearGuard HD cap is inserted into a liquid-filled catheter, chlorhexidine elutes from the rod into the catheter lock solution.
- The chlorhexidine coating dissolves to kill microorganisms on the inside and outside of the catheter hub.
- The existing catheter clamp holds the antimicrobial agent inside the catheter hub between treatments.
- ClearGuard HD caps are used in place of a standard cap or connector.
- Designed to meet your needs, ClearGuard HD caps can be used with heparin, citrate and saline solutions.

ClearGuard HD Caps are Clinically Proven
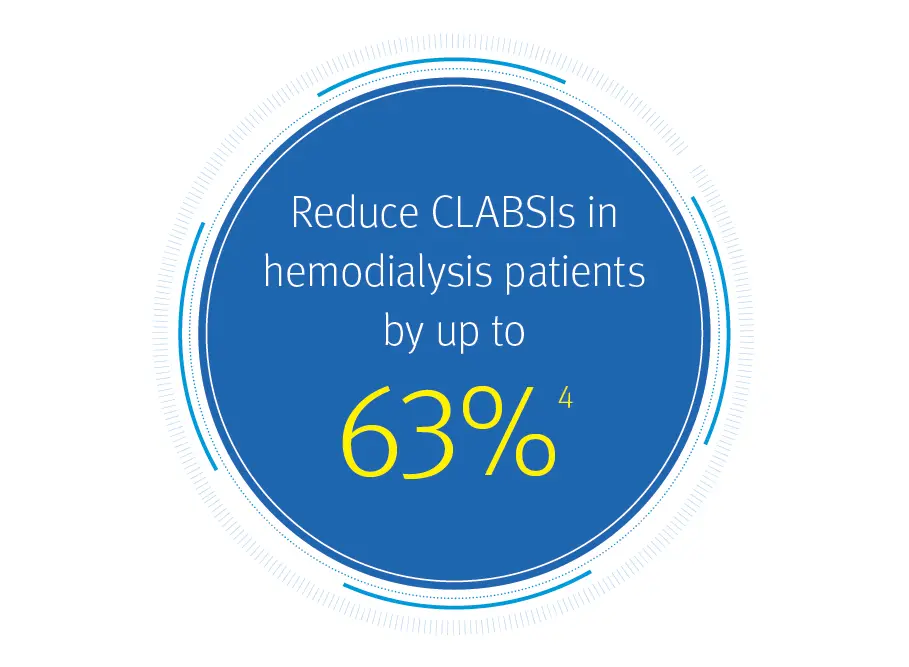
ClearGuard HD caps are clinically proven to reduce CLABSIs in hemodialysis catheter patients
Multiple large, prospective, cluster-randomized multicenter open-label trials demonstrated a significant reduction in the rate of positive blood cultures (PBCs) and CLABSIs using ClearGuard HD caps vs. control groups.
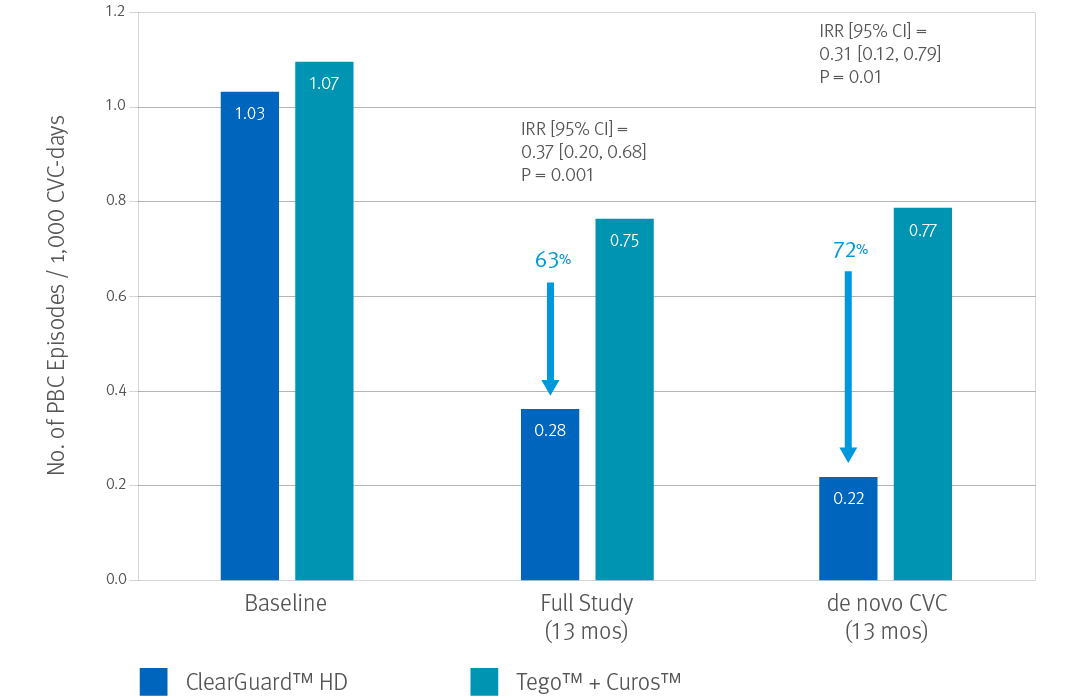
ClearGuard HD Caps vs. Tego™+ Curos™
Brunelli, SM et al. Cluster-randomized trial of devices to prevent catheter-related bloodstream infection. J Am Soc Nephrol 2018 Apr; 29(4):1336-1343.
- 13-month prospective, cluster-randomized, multicenter open-label trial
- 1671 patients (826 treatment, 845 control) accruing ~183,000 CVC days
- 40 centers across the US
- Primary endpoint was PBC rate as an indicator of BSI rate.
When considering the de novo subgroup (new CVCs), use of ClearGuard HD caps was associated with a 72% lower BSI rate.
Curos is a trademark of 3M Company.
JASN*
Cluster-Randomized Trial of Devices to
Prevent Catheter-Related Bloodstream Infections
*Chart is based on data contained in the article
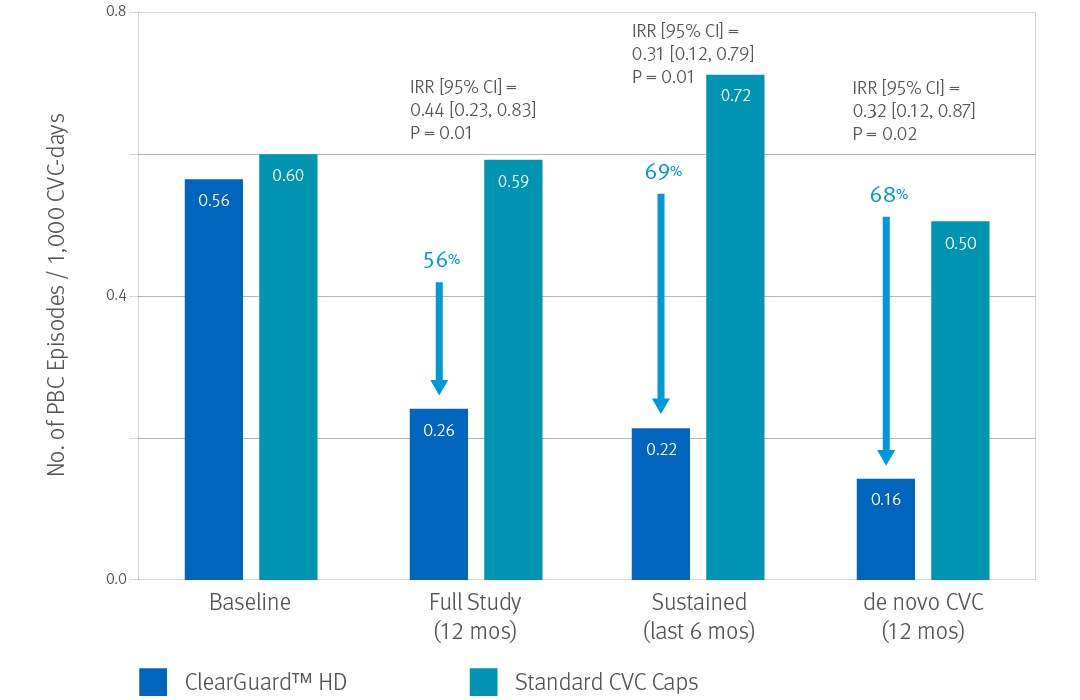
ClearGuard HD caps vs. standard
dialysis caps
Hymes, JL et al. Dialysis catheter-related bloodstream infections: a cluster-randomized trial of the ClearGuard HD antimicrobial barrier cap. Am J Kidney Dis. 2017; 69(2):220-227.
- 12-month prospective, cluster-randomized, multicenter, open-label comparative effectiveness trial in hemodialysis patients with central venous catheters
- 2470 patients (1245 treatment, 1225 control) accruing ~350,000 CVC days
- 40 centers across the US
- Primary endpoint was PBC rate as an indicator of BSI rate.
Results: Use of the ClearGuard HD caps for 12 months was associated with a 56% lower BSI rate vs. use of standard caps. When considering sustained use (defined as 6 months of the study), the intervention vs. control was associated with a 69% lower BSI rate.
AJKD*
Dialysis Catheter-Related Bloodstream Infections:
A Cluster-Randomized Trial of the Clearguard HD Antimicrobial Barrier Cap
*Chart is based on data contained in the article
A New Standard of Care

INS SOP, 27. Vascular Access and Hemodialysis, Section C3, For patients receiving hemodialysis through a CVAD, consider the use of an antimicrobial barrier cap as a strategy to reduce bloodstream infections. (Practice Recommendations)
INS SOP, 47. Vascular Access Device-Related Infection, Section J, For patients receiving outpatient dialysis through a central venous catheter, consider the use of an antimicrobial barrier cap as a strategy to reduce bloodstream infection. (Practice Recommendations)

Recommended in NKF's KDOQI Clinical Practice Guideline for Vascular Access: 20197
21.3 KDOQI considers it reasonable to use an antimicrobial barrier cap to help reduce CRBSI in high-risk patients or facilities; the choice of connector should be based on clinician’s discretion and best clinical judgment. (Expert Opinion)

Special Report published by Global Business Media exclusively features ClearGuard HD, titled: Reducing Catheter Related Bloodstream Infections in Hemodialysis Patients

Recommended in the UK's NICE Medical Technologies Guidance for haemodialysis catheter-related bloodstream infections: 20218
ClearGuard HD antimicrobial barrier caps used by leading hospitals and clinics
For years, dialysis staff at hospitals and clinics have focused on educational initiatives to reduce bloodstream infections in hemodialysis patients with limited impact. ClearGuard HD caps succeed in reducing infections by killing bacteria where bloodstream infections start—inside the hemodialysis catheter hub. With over 50 million treatments at 5,200 outpatient dialysis clinics and over 1,700 hospitals, ClearGuard HD caps are becoming an increasingly important part of hemodialysis infection control best practices.
Contact Us Today
Start protecting your patients today and leave no hemodialysis CVC unprotected
Contact us today to find out how ClearGuard HD caps can play a large role in your infection control best practices.

Related Products
Product Inquiry
Please enter your details into the following form.
References
-
Nguyen OB, Shugart A, Lines C, Shah AB, Edwards J, Pollock D, Sievert D, Patel PR. National healthcare safety network (NHSN) dialysis event surveillance report for 2014. Clin J Am Soc Nephrol 12(7), 1139-1146, 2017.
-
United States Renal Data System. 2018 USRDS annual report: Epidemiology of kidney disease in the Unites States. National Institutes of Health, Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2018.
-
Results. Content last reviewed November 2017. Agency for Healthcare Research and Quality, Rockville, MD. https://www.ahrq.gov/hai/pfp/haccost2017-results.html
-
Brunelli, SM et al. Cluster-randomized trial of devices to prevent catheter-related bloodstream infection. J Am Soc Nephrol. 2018 Apr,29(4):1336-1343.
-
Hymes, JL et al. Dialysis catheter-related bloodstream infections: a cluster-randomized trial of the ClearGuard HD antimicrobial barrier cap. Am J Kidney Dis. 2017 Feb;69(2)220-227.
-
INS Standards of Practice
-
Lok CE, Huber TS, Lee T, et al. KDOQI Vascular Access Guideline Work Group. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75(4)(suppl 2):S1-S164.
-
© NICE 2021 ClearGuard HD antimicrobial barrier caps for preventing haemodialysis catheter-related bloodstream infections. Medical technologies guidance [MTG62]. Available from www.nice.org.uk/guidance/mtg62/chapter/1-Recommendations. All rights reserved. Subject to notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.