Select Safely
IV Containers and the Updated USP <797> Standards
ICU Medical Blog
March 1, 2024
Protect patients by protecting their medications
When it comes to hazardous drug handling, safety never stops. For example, the ever-evolving landscape of safe handling guidelines goes beyond staff protection in USP <797>, which aims to safeguard the integrity and efficacy of life-saving medications prepared during the hazardous drug process.
So, how will that impact your facility? Maintaining compliance means constantly adapting policies and processes to keep your people and your patients safe. So, it’s time to act.
If you’re already in the process of navigating the new USP standards, great. If not, don’t panic—just take it one step at a time.
For right now, let’s focus on the USP requirements for selecting intravenous (IV) containers able to handle the challenges of hazardous drug compatibility—and the importance of giving prepared medications additional layers of security.
Choose your container wisely
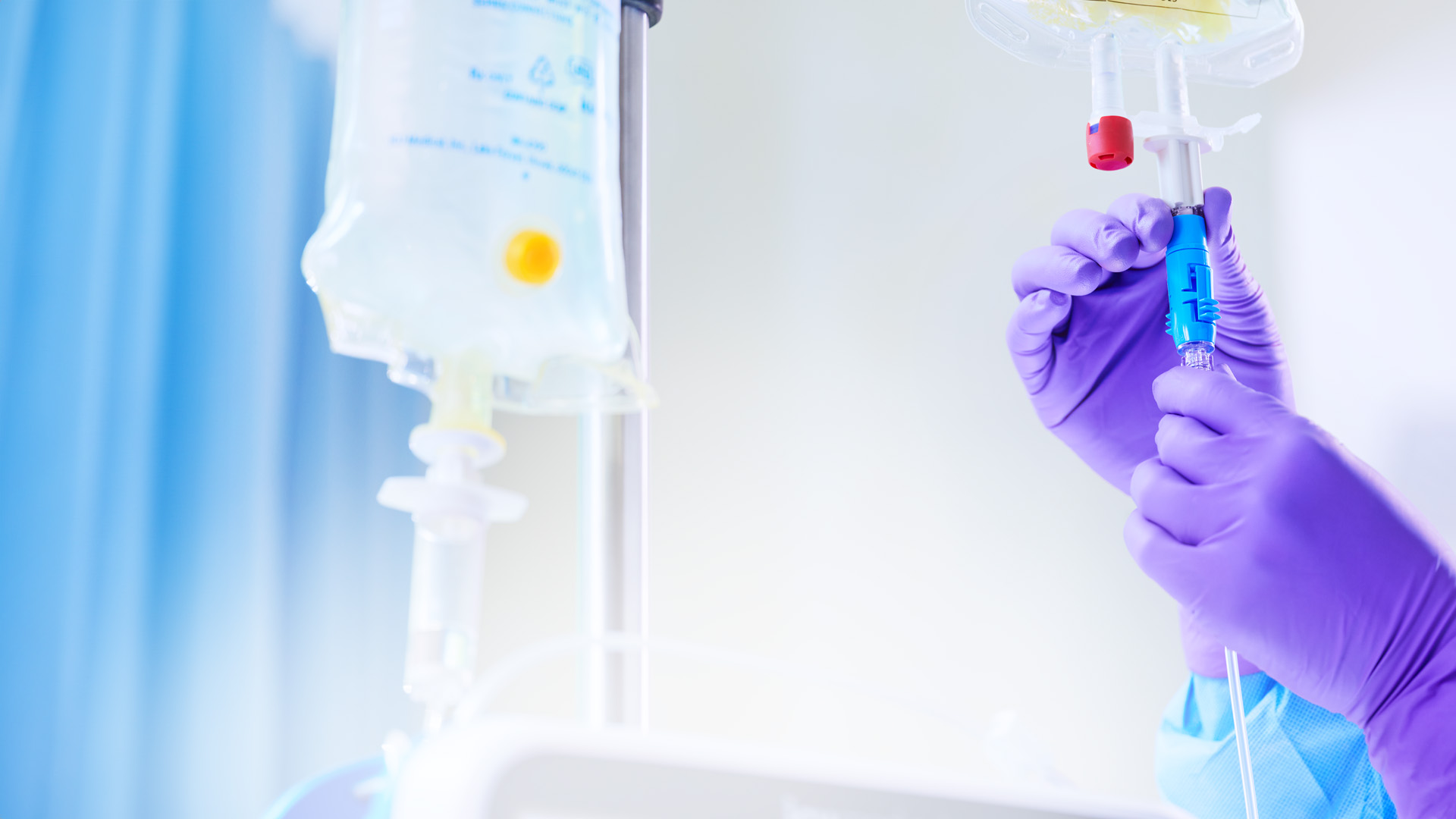
Selecting the right IV container is crucial for administering hazardous medication like chemotherapy agents since leaching and sorption can significantly compromise drug integrity and efficacy.
Leaching occurs when certain ingredients in the drug solutions, like surfactants, cause the release of plasticizers from the IV container.
For instance, Paclitaxel, a fat-based carrier, can leach DEHP (Di(2-ethylhexyl)phthalate) from PVC (polyvinyl chloride) containers and reduce the solubility of drugs during administration.1
Moreover, leached DEHP not only compromises the drug's solubility but also affects human metabolism, leading to decreased efficacy due to sorption on the inner surface of the PVC infusion container.1
To mitigate these issues, it’s a good idea to consult drug manufacturers or approved resources to verify specific drug compatibility, since some non-DEHP containers may have a PVC base with a different plasticizer.
This check will help you reduce the risk of leaching and sorption while helping ensure that a drug maintains its pharmaceutical efficacy.
Additionally, go for IV containers with an overwrap whenever possible, as it offers an extra layer of protection and further safeguards the drug’s integrity.
Double down on safety with additional layers of protection
Even if you’ve chosen the right IV container, that’s just one (albeit very important) layer of protection. Protecting the integrity of medications also includes preventing instances of unauthorized access like drug diversion and adulteration where healthcare staff illegally distribute, abuse, or alter medications, posing significant risk to patients.
The safety of the nation’s pharmaceutical supply is critically important.
Intentional adulteration is a serious risk at any facility that handles drugs. A recent example occurred in 2022 when a Dallas, Texas, anesthesiologist allegedly injected nerve-blocking agents and other drugs into patient IV bags at a local surgery center; multiple cardiac emergencies and at least one death resulted.2
To keep cases like this from repeating, steps to combat adulteration and drug diversion require swift, consistent efforts to maintain the integrity of a drug product and ultimately patient safety.
For example, USP <797> recognizes the additional security tamper-evident caps can provide to ensure drug integrity. These caps provide a visible assurance that a drug mixture is complete and that no further manipulation of the medication port has occurred.
Protecting drug integrity is paramount in protecting your patients—and an area where going above and beyond minimum standards is encouraged.
Start with an assessment
But, where to begin? Navigating the intricacies of the updated USP standard requires a comprehensive understanding of hazardous drug workflows and safety concerns. And IV containers with layers of protection are just one component of a much larger USP compliance plan.
In many cases, a third-party assessment can offer valuable insights, providing an impartial and expert evaluation of your current practices—which could be a meaningful starting point for your own plan.
ICU Medical offers a complimentary assessment to assist healthcare providers in reducing exposure risks and achieving USP <797> compliance.
Related Articles
References
- Park KH, Chung DJ. Stability study of docetaxel solution (0.9%, saline) using non-PVC and PVC tubes for intravenous administration. Biomater Res. 2015 Jan 28;19:2. doi: 10.1186/s40824-014-0023-x.
- Texas anesthesiologist arrested on criminal charges related to alleged tampering with IV bags implicated in death, surgical emergencies [press release]. United States Attorney’s Office, Northern District of Texas. September 15, 2022. https://www.justice.gov/usao-ndtx/pr/texas-anesthesiologist-arrested-criminal-charges-related-alleged-tampering-iv-bags. Accessed January 17, 2024.